What Masses of Glaubers Salt Must Be Used to Provide 1 Mol of Sodium Sulfate
| |||
| Names | |||
|---|---|---|---|
| Other names Sodium sulphate | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.028.928 | ||
| E number | E514(i) (acerbity regulators, ...) | ||
| PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical formula | NatwoSO4 | ||
| Molar mass | 142.04 g/mol (anhydrous) 322.twenty g/mol (decahydrate) | ||
| Appearance | white crystalline solid hygroscopic | ||
| Smell | odorless | ||
| Density | two.664 g/cm3 (anhydrous) i.464 thou/cm3 (decahydrate) | ||
| Melting point | 884 °C (1,623 °F; 1,157 Chiliad) (anhydrous) 32.38 °C (decahydrate) | ||
| Boiling point | i,429 °C (2,604 °F; 1,702 K) (anhydrous) | ||
| Solubility in water | anhydrous: 4.76 g/100 mL (0 °C) 28.1 g/100 mL (25 °C)[ane] 42.7 thousand/100 mL (100 °C) heptahydrate: 19.5 m/100 mL (0 °C) 44 m/100 mL (xx °C) | ||
| Solubility | insoluble in ethanol soluble in glycerol, water and hydrogen iodide | ||
| Magnetic susceptibility (χ) | −52.0·10−half-dozen cm3/mol | ||
| Refractive index (northward D) | 1.468 (anhydrous) 1.394 (decahydrate) | ||
| Construction | |||
| Crystal construction | orthorhombic (anhydrous)[2] monoclinic (decahydrate) | ||
| Pharmacology | |||
| ATC code | A06AD13 (WHO) A12CA02 (WHO) | ||
| Hazards | |||
| Occupational rubber and health (OHS/OSH): | |||
| Main hazards | Irritant | ||
| NFPA 704 (burn diamond) | i 0 0 | ||
| Flash indicate | Non-flammable | ||
| Rubber data sheet (SDS) | ICSC 0952 | ||
| Related compounds | |||
| Other anions | Sodium selenate Sodium tellurate | ||
| Other cations | Lithium sulfate Potassium sulfate Rubidium sulfate Caesium sulfate | ||
| Related compounds | Sodium bisulfate Sodium sulfite Sodium persulfate | ||
| Supplementary data page | |||
| Sodium sulfate (data page) | |||
| Except where otherwise noted, information are given for materials in their standard country (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula NaiiSO4 likewise as several related hydrates. All forms are white solids that are highly soluble in water. With an almanac production of half-dozen meg tonnes, the decahydrate is a major article chemical product. It is mainly used as a filler in the manufacture of powdered abode laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.[iii]
Forms [edit]
- Anhydrous sodium sulfate, known as the rare mineral thenardite, used as a drying amanuensis in organic synthesis.
- Heptahydrate sodium sulfate, a very rare course.
- Decahydrate sodium sulfate, known as the mineral mirabilite, widely used by chemical industry. It is also known as Glauber's salt.
History [edit]
The decahydrate of sodium sulfate is known every bit Glauber'southward common salt after the Dutch/German chemist and apothecary Johann Rudolf Glauber (1604–1670), who discovered information technology in Austrian jump h2o in 1625. He named it sal mirabilis (miraculous salt), because of its medicinal properties: the crystals were used equally a general-purpose laxative, until more than sophisticated alternatives came well-nigh in the 1900s.[4] [v]
In the 18th century, Glauber'south salt began to be used as a raw textile for the industrial production of soda ash (sodium carbonate), past reaction with potash (potassium carbonate). Demand for soda ash increased, and the supply of sodium sulfate had to increase in line. Therefore, in the 19th century, the large-scale Leblanc process, producing constructed sodium sulfate equally a cardinal intermediate, became the principal method of soda-ash production.[half-dozen]
Chemical backdrop [edit]
Sodium sulfate is a typical electrostatically bonded ionic sulfate. The existence of free sulfate ions in solution is indicated by the easy formation of insoluble sulfates when these solutions are treated with Batwo+ or Atomic number 822+ salts:
- Na2SOiv + BaCltwo → ii NaCl + BaSO4
Sodium sulfate is unreactive toward most oxidizing or reducing agents. At loftier temperatures, it can be converted to sodium sulfide by carbothermal reduction (aka thermo-chemical sulfate reduction (TSR), high temperature heating with charcoal, etc.):[7]
- Na2And so4 + 2 C → Na2South + 2 CO2
This reaction was employed in the Leblanc process, a defunct industrial route to sodium carbonate.
Sodium sulfate reacts with sulfuric acid to give the acrid salt sodium bisulfate:[viii] [nine]
- Na2And then4 + H2And so4 ⇌ two NaHSOiv
Sodium sulfate displays a moderate trend to form double salts. The merely alums formed with common trivalent metals are NaAl(SOiv)two (unstable above 39 °C) and NaCr(And soiv)2, in contrast to potassium sulfate and ammonium sulfate which course many stable alums.[10] Double salts with some other alkaline sulfates are known, including Na2And then4·3K2Then4 which occurs naturally as the mineral aphthitalite. Formation of glaserite by reaction of sodium sulfate with potassium chloride has been used as the ground of a method for producing potassium sulfate, a fertiliser.[xi] Other double salts include 3Na2SOiv·CaSO4, 3Na2SO4·MgSOiv (vanthoffite) and NaF·Na2SO4.[12]
Concrete properties [edit]
Sodium sulfate has unusual solubility characteristics in water.[13] Its solubility in water rises more than tenfold between 0 °C and 32.384 °C, where it reaches a maximum of 49.vii chiliad/100 mL. At this point the solubility bend changes slope, and the solubility becomes almost independent of temperature. This temperature of 32.384 °C, respective to the release of crystal h2o and melting of the hydrated salt, serves as an accurate temperature reference for thermometer calibration.
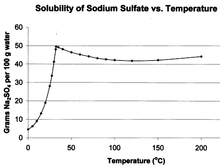
Temperature dependence of NaiiSO4 solubility in water
Structure [edit]
Crystals of the decahydrate consist of [Na(OHii)6]+ ions with octahedral molecular geometry. These octahedra share edges such that viii of the x h2o molecules are jump to sodium and two others are interstitial, being hydrogen-bonded to sulfate. These cations are linked to the sulfate anions by hydrogen bonds. The Na–O distances are about 240 pm.[fourteen] Crystalline sodium sulfate decahydrate is likewise unusual amid hydrated salts in having a measurable rest entropy (entropy at absolute cypher) of 6.32 J/(Thousand·mol). This is ascribed to its ability to distribute water much more than rapidly compared to most hydrates.[15]
Product [edit]
The world product of sodium sulfate, almost exclusively in the form of the decahydrate amounts to approximately 5.5 to 6 million tonnes annually (Mt/a). In 1985, production was 4.5 Mt/a, half from natural sources, and half from chemical production. After 2000, at a stable level until 2006, natural production had increased to 4 Mt/a, and chemic production decreased to ane.5 to 2 Mt/a, with a full of 5.5 to half dozen Mt/a.[xvi] [17] [eighteen] [19] For all applications, naturally produced and chemically produced sodium sulfate are practically interchangeable.
Natural sources [edit]
Two thirds of the world'southward production of the decahydrate (Glauber'southward common salt) is from the natural mineral course mirabilite, for example as constitute in lake beds in southern Saskatchewan. In 1990, Mexico and Spain were the world'southward principal producers of natural sodium sulfate (each effectually 500,000 tonnes), with Russia, United States and Canada around 350,000 tonnes each.[17] Natural resources are estimated at over 1 billion tonnes.[16] [17]
Major producers of 200,000 to one,500,000 tonnes/year in 2006 included Searles Valley Minerals (California, United states), Airborne Industrial Minerals (Saskatchewan, Canada), Química del Rey (Coahuila, United mexican states), Minera de Santa Marta and Criaderos Minerales Y Derivados, also known as Grupo Crimidesa (Burgos, Spain), Minera de Santa Marta (Toledo, Spain), Sulquisa (Madrid, Spain), Chengdu Sanlian Tianquan Chemical (Tianquan County, Sichuan, China), Hongze Yinzhu Chemical Grouping (Hongze Commune, Jiangsu, China), Nafine Chemical Manufacture Grouping (Shanxi, China), Sichuan Province Chuanmei Mirabilite (万胜镇, Dongpo District, Meishan, Sichuan, Mainland china), and Kuchuksulphat JSC (Altai Krai, Siberia, Russia).[16] [eighteen]
Anhydrous sodium sulfate occurs in barren environments equally the mineral thenardite. Information technology slowly turns to mirabilite in damp air. Sodium sulfate is too institute as glauberite, a calcium sodium sulfate mineral. Both minerals are less mutual than mirabilite.[ citation needed ]
Chemic industry [edit]
Nigh one 3rd of the world's sodium sulfate is produced as by-production of other processes in chemic industry. Most of this product is chemically inherent to the master procedure, and only marginally economical. By attempt of the industry, therefore, sodium sulfate product as by-production is declining.
The almost important chemic sodium sulfate production is during hydrochloric acid production, either from sodium chloride (table salt) and sulfuric acrid, in the Mannheim process, or from sulfur dioxide in the Hargreaves process.[twenty] The resulting sodium sulfate from these processes is known as salt cake .
- Mannheim: 2 NaCl + H2Then4 → two HCl + Na2SO4
- Hargreaves: 4 NaCl + ii SO2 + Oii + 2 HiiO → 4 HCl + 2 NatwoAnd so4
The 2d major product of sodium sulfate are the processes where surplus sodium hydroxide is neutralised past sulfuric acrid, as practical on a large scale in the product of rayon. This method is also a regularly applied and convenient laboratory training.
- 2 NaOH(aq) + HtwoAnd so4(aq) → Na2SO4(aq) + 2 H2O(l) ΔH = -112.5 kJ (highly exothermic)
In the laboratory it can also be synthesized from the reaction betwixt sodium bicarbonate and magnesium sulfate.
- 2 NaHCO3 + MgSOfour → Na2Soiv + Mg(OH)2 + 2 CO2
Yet, as commercial sources are readily available, laboratory synthesis is not practised often. Formerly, sodium sulfate was also a by-product of the manufacture of sodium dichromate, where sulfuric acid is added to sodium chromate solution forming sodium dichromate, or subsequently chromic acid. Alternatively, sodium sulfate is or was formed in the production of lithium carbonate, chelating agents, resorcinol, ascorbic acid, silica pigments, nitric acrid, and phenol.[16]
Bulk sodium sulfate is ordinarily purified via the decahydrate form, since the anhydrous form tends to attract iron compounds and organic compounds. The anhydrous form is easily produced from the hydrated form past gentle warming.
Major sodium sulfate by-product producers of 50–80 Mt/a in 2006 include Elementis Chromium (chromium industry, Castle Hayne, NC, Usa), Lenzing AG (200 Mt/a, rayon industry, Lenzing, Austria), Addiseo (formerly Rhodia, methionine industry, Les Roches-Roussillon, French republic), Elementis (chromium manufacture, Stockton-on-Tees, United kingdom of great britain and northern ireland), Shikoku Chemicals (Tokushima, Nihon) and Visko-R (rayon industry, Russian federation).[16]
Applications [edit]
Sodium sulfate used to dry an organic liquid. Here clumps class, indicating the presence of h2o in the organic liquid.
By farther application of sodium sulfate the liquid may be brought to dryness, indicated here by the absence of clumping.
Commodity industries [edit]
With US pricing at $30 per tonne in 1970, up to $90 per tonne for common salt block quality, and $130 for improve grades, sodium sulphate is a very cheap fabric. The largest utilize is every bit filler in powdered home laundry detergents, consuming approx. 50% of globe production. This use is waning as domestic consumers are increasingly switching to compact or liquid detergents that practice not include sodium sulfate.[16]
Another formerly major use for sodium sulfate, notably in the The states and Canada, is in the Kraft procedure for the manufacture of wood pulp. Organics present in the "black liquor" from this process are burnt to produce rut, needed to drive the reduction of sodium sulfate to sodium sulfide. However, due to advances in the thermal efficiency of the Kraft recovery process in the early 1960s, more efficient sulfur recovery was achieved and the demand for sodium sulfate makeup was drastically reduced.[21] Hence, the utilise of sodium sulfate in the US and Canadian lurid industry declined from i,400,000 tonnes per twelvemonth in 1970 to only approx. 150,000 tonnes in 2006.[16]
The drinking glass manufacture provides another pregnant application for sodium sulfate, as second largest application in Europe. Sodium sulfate is used every bit a fining agent, to assistance remove pocket-sized air bubbles from molten glass. It fluxes the drinking glass, and prevents scum formation of the glass melt during refining. The glass industry in Europe has been consuming from 1970 to 2006 a stable 110,000 tonnes annually.[16]
Sodium sulfate is important in the manufacture of textiles, particularly in Japan, where it is the largest application. Sodium sulfate is added to increment the ionic force of the solution and then helps in "levelling", reducing negative electrical charges on textile fibres so that dyes can penetrate evenly (run across the theory of the diffuse double layer (DDL) elaborated by Gouy and Chapman). Unlike the alternative sodium chloride, it does not corrode the stainless steel vessels used in dyeing. This application in Japan and US consumed in 2006 approximately 100,000 tonnes.[16]
Food industry [edit]
Sodium sulfate is used equally a diluent for nutrient colours.[22] It is known equally Eastward number additive E514.
Thermal storage [edit]
The high rut storage capacity in the phase change from solid to liquid, and the advantageous phase modify temperature of 32 °C (90 °F) makes this material especially appropriate for storing low class solar rut for later release in space heating applications. In some applications the cloth is incorporated into thermal tiles that are placed in an attic space while in other applications the common salt is incorporated into cells surrounded by solar–heated water. The stage alter allows a substantial reduction in the mass of the cloth required for effective estrus storage (the estrus of fusion of sodium sulfate decahydrate is 82 kJ/mol or 252 kJ/kg[23]), with the farther advantage of a consistency of temperature as long as sufficient material in the advisable phase is available.
For cooling applications, a mixture with mutual sodium chloride salt (NaCl) lowers the melting point to 18 °C (64 °F). The rut of fusion of NaCl·Na2And thenfour·10H2O, is actually increased slightly to 286 kJ/kg.[24]
Small-scale applications [edit]
In the laboratory, anhydrous sodium sulfate is widely used every bit an inert drying agent, for removing traces of water from organic solutions.[25] It is more efficient, but slower-acting, than the similar agent magnesium sulfate. Information technology is just effective beneath about 30 °C, simply information technology can be used with a variety of materials since information technology is chemically adequately inert. Sodium sulfate is added to the solution until the crystals no longer dodder together; the two video clips (come across above) demonstrate how the crystals clump when still wet, but some crystals flow freely in one case a sample is dry.
Glauber'due south salt, the decahydrate, is used every bit a laxative. It is effective for the removal of certain drugs such as paracetamol (acetaminophen) from the trunk, for case, after an overdose.[26] [27]
In 1953, sodium sulfate was proposed for heat storage in passive solar heating systems. This takes advantage of its unusual solubility properties, and the loftier heat of crystallisation (78.two kJ/mol).[28]
Other uses for sodium sulfate include de-frosting windows, starch manufacture, as an condiment in rug fresheners, and as an condiment to cattle feed.
At least one company, Thermaltake, makes a laptop computer chill mat (iXoft Notebook Cooler) using sodium sulfate decahydrate inside a quilted plastic pad. The material slowly turns to liquid and recirculates, equalizing laptop temperature and acting as an insulation.[29]
Condom [edit]
Although sodium sulfate is more often than not regarded as non-toxic,[22] information technology should be handled with care. The dust can crusade temporary asthma or eye irritation; this hazard can be prevented by using eye protection and a paper mask. Ship is non limited, and no Gamble Phrase or Prophylactic Phrase applies.[30]
References [edit]
- ^ National Center for Biotechnology Information. PubChem Chemical compound Summary for CID 24436, Sodium sulfate. https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-sulfate. Accessed Nov. 2, 2020.
- ^ Zachariasen, Due west. H.; Ziegler, K. Due east. (1932). "The crystal structure of anhydrous sodium sulfate Na2SO4". Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie. Wiesbaden: Akademische Verlagsgesellschaft. 81 (1–6): 92–101. doi:x.1524/zkri.1932.81.one.92. S2CID 102107891.
{{cite journal}}: CS1 maint: multiple names: authors listing (link) - ^ Helmold Plessen (2000). "Sodium Sulfates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_355. ISBN978-3527306732.
- ^ Szydlo, Zbigniew (1994). H2o which does not wet hands: The Abracadabra of Michael Sendivogius. London–Warsaw: Smooth University of Sciences.
- ^ Westfall, Richard Due south. (1995). "Glauber, Johann Rudolf". The Galileo Project. Archived from the original on 2011-xi-eighteen.
- ^ Aftalion, Fred (1991). A History of the International Chemical Industry. Philadelphia: University of Pennsylvania Press. pp. 11–16. ISBN978-0-8122-1297-6.
- ^ Handbook of Chemistry and Physics (71st ed.). Ann Arbor, Michigan: CRC Press. 1990. ISBN9780849304712.
- ^ The Merck Index (7th ed.). Rahway, New Jersey, US: Merck & Co. 1960.
- ^ Nechamkin, Howard (1968). The Chemistry of the Elements . New York: McGraw-Loma.
- ^ Lipson, Henry; Beevers, C. A. (1935). "The Crystal Construction of the Alums". Proceedings of the Majestic Society A. 148 (865): 664–80. Bibcode:1935RSPSA.148..664L. doi:10.1098/rspa.1935.0040.
- ^ Garrett, Donald E. (2001). Sodium sulfate : handbook of deposits, processing, properties, and use. San Diego: Academic Press. ISBN978-0-12-276151-v.
- ^ Mellor, Joseph William (1961). Mellor's Comprehensive Treatise on Inorganic and Theoretical Chemistry. Vol. II (new impression ed.). London: Longmans. pp. 656–673. ISBN978-0-582-46277-9.
- ^ Linke, Westward. F.; A. Seidell (1965). Solubilities of Inorganic and Metal Organic Compounds (4th ed.). Van Nostrand. ISBN978-0-8412-0097-v.
- ^ Helena Due west. Ruben, David H. Templeton, Robert D. Rosenstein, Ivar Olovsson, "Crystal Structure and Entropy of Sodium Sulfate Decahydrate", J. Am. Chem. Soc. 1961, volume 83, pp. 820–824. doi:10.1021/ja01465a019.
- ^ Brodale, G.; W. F. Giauque (1958). "The Heat of Hydration of Sodium Sulfate. Depression Temperature Heat Capacity and Entropy of Sodium Sulfate Decahydrate". Journal of the American Chemic Society. lxxx (9): 2042–2044. doi:10.1021/ja01542a003.
- ^ a b c d eastward f m h i Suresh, Bala; Kazuteru Yokose (May 2006). Sodium sulfate. CEH Marketing Research Written report. Zurich: Chemical Economic Handbook SRI Consulting. pp. 771.1000A–771.1002J. Archived from the original on 2007-03-14.
- ^ a b c "Statistical compendium Sodium sulfate". Reston, Virginia: United states Geological Survey, Minerals Information. 1997. Archived from the original on 2007-03-07. Retrieved 2007-04-22 .
- ^ a b The economic science of sodium sulphate (Eighth ed.). London: Roskill Information Services. 1999.
- ^ The sodium sulphate business. London: Chem Systems International. November 1984.
- ^ Butts, D. (1997). Kirk-Othmer Encyclopedia of Chemic Technology. Vol. v22 (quaternary ed.). pp. 403–411.
- ^ Smook, Gary (2002). Handbook for Pulp and Paper Technologists. p. 143. Archived from the original on 2016-08-07.
- ^ a b "Sodium sulfate (WHO Food Additives Series 44)". Globe Health Organization. 2000. Archived from the original on 2007-09-04. Retrieved 2007-06-06 .
- ^ "Archived copy" (PDF). Archived (PDF) from the original on 2015-09-24. Retrieved 2014-06-19 .
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "Archived copy" (PDF). Archived (PDF) from the original on 2015-09-24. Retrieved 2014-06-19 .
{{cite web}}: CS1 maint: archived copy as championship (link) p.8 - ^ Vogel, Arthur I.; B.V. Smith; Due north.One thousand. Waldron (1980). Vogel's Elementary Practical Organic Chemistry 1 Preparations (3rd ed.). London: Longman Scientific & Technical.
- ^ Cocchetto, D.M.; G. Levy (1981). "Absorption of orally administered sodium sulfate in humans". J Pharm Sci. 70 (iii): 331–3. doi:ten.1002/jps.2600700330. PMID 7264905.
- ^ Prescott, L. F.; Critchley, J. A. J. H. (1979). "The Treatment of Acetaminophen Poisoning". Almanac Review of Pharmacology and Toxicology. 23: 87–101. doi:10.1146/annurev.pa.23.040183.000511. PMID 6347057.
- ^ Telkes, Maria (1953). Improvements in or relating to a device and a limerick of matter for the storage of oestrus. British Patent No. GB694553.
- ^ "IXoft Specification". Thermaltake Technology Co., Ltd. Archived from the original on 2016-03-12. Retrieved 2015-08-15 .
- ^ "MSDS Sodium Sulfate Anhydrous". James T Baker. 2006. Archived from the original on 2003-06-19. Retrieved 2007-04-21 .
External links [edit]
- Calculators: surface tensions, and alsodensities, molarities and molalities of aqueous sodium sulfate
Source: https://en.wikipedia.org/wiki/Sodium_sulfate


