Harris Find Cu2 in a Solution Saturated
Electrochemical Reactions
Electrical Work From Spontaneous Oxidation-Reduction Reactions
The following rule can be used to predict whether an oxidation-reduction reaction should occur. Oxidation-reduction reactions should occur when they convert the stronger of a pair of oxidizing agents and the stronger of a pair of reducing agents into a weaker oxidizing agent and a weaker reducing agent.
We can test this prediction by adding a few chunks of mossy zinc to a beaker of concentrated hydrochloric acid. Within a few minutes, the zinc metal dissolves, and significant amounts of hydrogen gas are liberated.
The reaction in Practice Problem 1 has some of the characteristic features of oxidation-reduction reactions.
- It is exothermic, in this case giving off 153.89 kilojoules per mole of zinc consumed.
- The equilibrium constant for the reaction is very large (Kc = 6 x 1025), and chemists often write the equation for this reaction as if essentially all of the reactants were converted to products.
Zn(s) + 2 H+(aq)
Zn2+(aq) + H2(g)
- It can be formally divided into separate oxidation and reduction half-reactions.
- By separating the two half-reactions, the energy given off by this reaction can be used to do work.
According to the first law of thermodynamics, the energy given off in a chemical reaction can be converted into heat, work, or a mixture of heat and work. By running the half-reactions in separate containers, we can force the electrons to flow from the oxidation to the reduction half-reaction through an external wire, which allows us to capture as much as possible of the energy given off in the reaction as electrical work.
We can start by immersing a strip of zinc metal into a 1 M Zn2+ ion solution, as shown in the figure below. We then immerse a piece of platinum wire in a second beaker filled with 1 M HCl and bubble H2 gas over the Pt wire. Finally, we connect the zinc metal and platinum wire to form an electric circuit.

We've now made a system in which electrons can flow from one half-reaction, or half-cell, to another. The same driving force that makes zinc metal react with acid when the two are in contact should operate in this system. Zinc atoms on the metal surface lose electrons to form Zn2+ ions, which go into solution.
| Oxidation: | Zn | | Zn2+ + 2 e- |
The electrons given off in this half-reaction flow through the circuit and eventually accumulate on the platinum wire to give this wire a net negative charge. The H+ ions from the hydrochloric acid are attracted to this negative charge and migrate toward the platinum wire. When the H+ ions touch the platinum wire, they pick up electrons to form hydrogen atoms, which immediately combine to form H2 molecules.
| Reduction: | 2 H+ + 2 e- | | H2 |
The oxidation of zinc metal releases Zn2+ ions into the Zn/Zn2+ half-cell. This half-cell therefore picks up a positive charge that interferes with the transfer of more electrons. The reduction of H+ ions in the H2/H+ half-cell leads to a net negative charge as these H+ ions are removed from the solution. This negative charge also interferes with the transfer of more electrons.
To overcome this problem, we complete the circuit by adding a U-tube filled with a saturated solution of a soluble salt such as KCl. Negatively charged Cl- ions flow out of one end of the U-tube to balance the positive charge on the Zn2+ ions created in one half-cell. Positively charged K+ ions flow out of the other end of the tube to replace the H+ ions consumed in the other half cell. The U-tube is called a salt bridge, because it contains a solution of a salt that literally serves as a bridge to complete the electric circuit.
![]()
Voltaic Cells
Electrochemical cells that use an oxidation-reduction reaction to generate an electric current are known as galvanic or voltaic cells. Because the potential of these cells to do work by driving an electric current through a wire is measured in units of volts, we will refer to the cells that generate this potential from now on as voltaic cells.
Let's take another look at the voltaic cell in the figure below.

Within each half-cell, reaction occurs on the surface of the metal electrode. At the zinc electrode, zinc atoms are oxidized to form Zn2+ ions, which go into solution. The electrons liberated in this reaction flow through the zinc metal until they reach the wire that connects the zinc electrode to the platinum wire. They then flow through the platinum wire, where they eventually reduce an H+ ion in the neighboring solution to a hydrogen atom, which combines with another hydrogen atom to form an H2 molecule.
The electrode at which oxidation takes place in a electrochemical cell is called the anode. The electrode at which reduction occurs is called the cathode. The identity of the cathode and anode can be remembered by recognizing that positive ions, or cations, flow toward the cathode, while negative ions, or anions, flow toward the anode. In the voltaic cell shown above, H+ ions flow toward the cathode, where they are reduced to H2 gas. On the other side of the cell, Cl- ions are released from the salt bridge and flow toward the anode, where the zinc metal is oxidized.
![]()
Standard-State Cell Potentials for Voltaic Cells
The cell potential for a voltaic cell is literally the potential of the cell to do work on its surroundings by driving an electric current through a wire. By definition, one joule of energy is produced when one coulomb of electrical charge is transported across a potential of one volt.
![]()
The potential of a voltaic cell depends on the concentrations of any species present in solution, the partial pressures of any gases involved in the reaction, and the temperature at which the reaction is run. To provide a basis for comparing the results of one experiment with another, the following set of standard-state conditions for electrochemical measurements has been defined.
- All solutions are 1 M.
- All gases have a partial pressure of 0.1 MPa (0.9869 atm).
Although standard-state measurements can be made at any temperature, they are often taken at 25oC.
Cell potentials measured under standard-state conditions are represented by the symbol E o. The standard-state cell potential, E o, measures the strength of the driving force behind the chemical reaction. The larger the difference between the oxidizing and reducing strengths of the reactants and products, the larger the cell potential. To obtain a relatively large cell potential, we have to react a strong reducing agent with a strong oxidizing agent.
Example: The experimental value for the standard-state cell potential for the reaction between zinc metal and acid is 0.76 volts.
| Zn(s) + 2 H+(aq) | E o = 0.76 V |
The cell potential for this reaction measures the relative reducing power of zinc metal compared with hydrogen gas. But it doesn't tell us anything about the absolute value of the reducing power for either zinc metal or H2.
We therefore arbitrarily define the standard-state potential for the reduction of H+ ions to H2 gas as exactly zero volts.
| 2 H+ + 2 e- | E o = 0.000... V |
We will then use this reference point to calibrate the potential of any other half-reaction.
The key to using this reference point is recognizing that the overall cell potential for a reaction must be the sum of the potentials for the oxidation and reduction half-reactions.
Eo overall = E o ox + E o red
If the overall potential for the reaction between zinc and acid is 0.76 volts, and the half-cell potential for the reduction of H+ ions is 0 volts, then the half-cell potential for the oxidation of zinc metal must be 0.76 volts.
![]()
Predicting Spontaneous Redox Reactions From the Sign of Eo
The magnitude of the cell potential is a measure of the driving force behind a reaction. The larger the value of the cell potential, the further the reaction is from equilibrium. The sign of the cell potential tells us the direction in which the reaction must shift to reach equilibrium.
Consider the reaction between zinc and acid, for example.
| Zn(s) + 2 H+(aq) | E o = 0.76 V |
The fact that E o is positive tells us that when this system is present at standard-state conditions, it has to shift to the right to reach equilibrium. Reactions for which E o is positive therefore have equilibrium constants that favor the products of the reaction. It is tempting to describe these reactions as "spontaneous."
What happens to the cell potential when we reverse the direction in which a reaction is written? Turning the reaction around doesn't change the relative strengths of the oxidizing or reducing agents. The magnitude of the potential must remain the same. But turning the equation around changes the sign of the cell potential, and can therefore turn an unfavorable reaction into one that is spontaneous, or vice versa.
Use the standard-state cell potential for the following reaction
| Cu(s) + 2 H+(aq) | E o = -0.34 V |
to predict the standard-state cell potential for the opposite reaction.
| Cu2+(aq) + H2(g) | E o = ? |
Click here to check your answer to Practice Problem 3
![]()
Standard-State Reduction Half-Cell Potentials
The standard-state cell potentials for some common half-reactions are given in the table below.
Standard-State Reduction Potentials, Eo red
There is no need to remember that reducing agents become stronger toward the upper right corner of this table, or that the strength of the oxidizing agents increases toward the bottom left corner. All you have to do is remember some of the chemistry of the elements at the top and bottom of this table.
Take a look at the half-reaction at the top of the table.
| K+ + e- | E o red = -2.924 V |
What do we know about potassium metal? Potassium is one of the most reactive metals![]() it bursts into flame when added to water, for example. Furthermore, we know that metals are reducing agents in all of their chemical reactions. When we find potassium in this table, we can therefore conclude that it is listed among the strongest reducing agents.
it bursts into flame when added to water, for example. Furthermore, we know that metals are reducing agents in all of their chemical reactions. When we find potassium in this table, we can therefore conclude that it is listed among the strongest reducing agents.
Conversely, look at the last reaction in the table.
| F2 + 2 e- | E o red = 3.03 V |
Fluorine is the most electronegative element in the periodic table. It shouldn't be surprising to find that F2 is the strongest oxidizing agent in the above table.
Referring to either end of this table can also help you remember the sign convention for cell potentials. The previous section introduced the following rule: Oxidation-reduction reactions that have a positive overall cell potential are spontaneous. This is consistent with the data in the above table. We know that fluorine wants to gain electrons to form fluoride ions, and the half-cell potential for this reaction is positive.
| F2 + 2 e- | E o red = 3.03 V |
We also know that potassium is an excellent reducing agent. Thus, the potential for the reduction of K+ ions to potassium metal is negative
| K+ + e- | E o red = -2.924 V |
but the potential for the oxidation of potassium metal to K+ ions is positive.
| K | E o ox = -(-2.924 V) = 2.924 V |
![]()
Predicting Standard-State Cell Potentials
A voltaic cell stable enough to be used as a battery is called a Daniell cell. For our purposes, we will work with the idealized Daniell cell in the figure below.
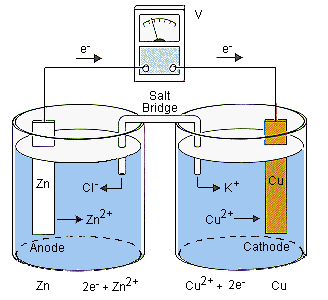
We can use the known values of the standard-state reduction potentials for the Cu/Cu2+ and Zn/Zn2+ half-cells to predict the overall potential for the Daniell cell and to determine which electrode is the anode and which is the cathode.
We start by writing a balanced chemical equation for the reaction that occurs in this cell. The table of standard-state reduction potentials suggests that zinc is a better reducing agent than copper and that the Cu2+ ion is a better oxidizing agent than the Zn2+ ion. The overall reaction therefore involves the reduction of Cu2+ ions by zinc metal.
| Zn(s) | + | Cu2+(aq) | | Zn2+(aq) | + | Cu(s) |
| stronger reducing agent | stronger oxidizing agent | weaker oxidizing agent | weaker reducing agent |
We then divide the reaction into separate oxidation and reduction half-reactions.
The potential for the reduction of Cu2+ ions to copper metal can be found in the table of standard-state reduction potentials. To find the potential for the oxidation of zinc metal, we have to reverse the sign on the potential for the Zn/Zn2+ couple in this table.
The overall potential for this cell is the sum of the potentials for the two half-cells.
Oxidation always occurs at the anode and reduction always occurs at the cathode of an electrochemical cell. The Zn/Zn2+ half-cell is therefore the anode, and the Cu2+/Cu half-cell is the cathode, as shown in the figure of the Daniell cell.
Use cell potential data to explain why copper metal does not dissolve in a typical strong acid, such as hydrochloric acid,
| Cu(s) + 2 H+(aq) | |
but will dissolve in 1 M nitric acid.
3 Cu(s) + 2 HNO3(aq) + 6 H+(aq) ![]() 3 Cu2+(aq) + 2 NO(g) + 4 H2O(l)
3 Cu2+(aq) + 2 NO(g) + 4 H2O(l)
Click here to check your answer to Practice Problem 5
The units of half-cell potentials are volts, not volts per mole or volts per electron. All we do when combining half-reactions is add the two half-cell potentials. We do not multiply these potentials by the integers used to balance the number of electrons transferred in the reaction.
![]()
Line Notation For Voltaic Cells
Voltaic cells can be described by a line notation based on the following conventions.
- A single vertical line indicates a change in state or phase.
- Within a half-cell, the reactants are listed before the products.
- Concentrations of aqueous solutions are written in parentheses after the symbol for the ion or molecule.
- A double vertical line is used to indicate the junction between the half-cells.
- The line notation for the anode (oxidation) is written before the line notation for the cathode (reduction).
The line notation for a standard-state Daniell cell is written as follows.
| Zn | | | Zn2+(1.0 M) | || | Cu2+(1.0 M) | | | Cu |
| anode (oxidation) | cathode (reduction) |
Electrons flow from the anode to the cathode in a voltaic cell. (They flow from the electrode at which they are given off to the electrode at which they are consumed.) Reading from left to right, this line notation therefore corresponds to the direction in which electrons flow.
![]()
The Nernst Equation
What happens when the Daniell cell is used to do work?
- The zinc electrode becomes lighter as zinc atoms are oxidized to Zn2+ ions, which go into solution.
- The copper electrode becomes heavier as Cu2+ ions in the solution are reduced to copper metal.
- The concentration of Zn2+ ions at the anode increases and the concentration of the Cu2+ ions at the cathode decreases.
- Negative ions flow from the salt bridge toward the anode to balance the charge on the Zn2+ ions produced at this electrode.
- Positive ions flow from the salt bridge toward the cathode to compensate for the Cu2+ ions consumed in the reaction.
An important property of the cell is missing from this list. Over a period of time, the cell runs down, and eventually has to be replaced. Let's assume that our cell is initially a standard-state cell in which the concentrations of the Zn2+ and Cu2+ ions are both 1 molar.
| Zn | | | Zn2+(1.0 M) | || | Cu2+(1.0 M) | | | Cu |
As the reaction goes forward![]() as zinc metal is consumed and copper metal is produced
as zinc metal is consumed and copper metal is produced![]() the driving force behind the reaction must become weaker. Therefore, the cell potential must become smaller.
the driving force behind the reaction must become weaker. Therefore, the cell potential must become smaller.
This raises an interesting question: When does the cell potential become zero?
The cell potential is zero if and only if the reaction is at equilibrium.
When the reaction is at equilibrium, there is no net change in the amount of zinc metal or copper ions in the system, so no electrons flow from the anode to the cathode. If there is no longer a net flow of electrons, the cell can no longer do electrical work. Its potential for doing work must therefore be zero.
In 1889 Hermann Walther Nernst showed that the potential for an electrochemical reaction is described by the following equation.
![]()
In the Nernst equation, E is the cell potential at some moment in time, E o is the cell potential when the reaction is at standard-state conditions, R is the ideal gas constant in units of joules per mole, T is the temperature in kelvin, n is the number of moles of electrons transferred in the balanced equation for the reaction, F is the charge on a mole of electrons, and Qc is the reaction quotient at that moment in time. The symbol ln indicates a natural logarithm, which is the log to the base e, where e is an irrational number equal to 2.71828...
Three terms in the Nernst equation are constants: R, T, and F. The ideal gas constant is 8.314 J/mol-K. The temperature is usually 25oC. The charge on a mole of electrons can be calculated from Avogadro's number and the charge on a single electron.

Substituting this information into the Nernst equation gives the following equation.
![]()
Three of the remaining terms in this equation are characteristics of a particular reaction: n, E o, and Qc .
Example: The standard-state potential for the Daniell cell is 1.10 V. Two moles of electrons are transferred from zinc metal to Cu2+ ions in the balanced equation for this reaction, so n is 2 for this cell. Because we never include the concentrations of solids in either reaction quotient or equilibrium constant expressions, Qc for this reaction is equal to the concentration of the Zn2+ ion divided by the concentration of the Cu2+ ion.
![]()
Substituting what we know about the Daniell cell into the Nernst equation gives the following result, which represents the cell potential for the Daniell cell at 25oC at any moment in time.
![]()
The figure below shows a plot of the potential for the Daniell cell as a function of the natural logarithm of the reaction quotient.

When the reaction quotient is very small, the cell potential is positive and relatively large. This isn't surprising, because the reaction is far from equilibrium and the driving force behind the reaction should be relatively large. When the reaction quotient is very large, the cell potential is negative. This means that the reaction would have to shift back toward the reactants to reach equilibrium.
Practice Problem 8 above raises an important point. The cell potential depends on the logarithm of the ratio of the concentrations of the products and the reactants. As a result, the potential of a cell or battery is more or less constant until virtually all of the reactants have been converted into products.
The Nernst equation can be used to calculate the potential of a cell that operates at non-standard-state conditions.
The Nernst equation can help us understand a popular demonstration that uses glucose, C6H12O6, to reduce Ag+ ions to silver metal, thereby forming a silver mirror on the inner surface of the container. The standard-state half-cell potential for the reduction of Ag+ ions is approximately 0.800 volt.
| Ag+ + e- | E o red = 0.800 V |
The standard-state half-cell potential for the oxidation of glucose is -0.050 V.
| C6H12O6 + H2O | E o ox = -0.050 V |
The overall standard-state cell potential for this reaction is therefore favorable.
The reaction isn't run under standard-state conditions, however. It takes place in a solution to which aqueous ammonia has been added. Most of the silver is therefore present as the Ag(NH3)2 + complex ion. This is important, because the half-cell potential for the reduction of this complex is considerably smaller than the potential for the reduction of the Ag+ ion.
| Ag(NH3)2 + + e- | E o red = 0.373 V |
This leads to a significant decrease in the overall cell potential for the reaction because the Ag(NH3)2 + ion is a much weaker oxidizing agent than the Ag+ ion.
The fact that this reaction is run in an aqueous ammonia solution also has an effect on the potential for the oxidation of glucose, because this half-reaction contains a pair of H+ ions.
C6H12O6 + H2O ![]() C6H12O7 + 2 H+ + 2 e-
C6H12O7 + 2 H+ + 2 e-
The half-cell potential for this reaction therefore depends on the pH of the solution. Because two H+ ions are given off when glucose is oxidized, the reaction quotient for this reaction depends on the square of the H+ ion concentration. A change in this solution from standard-state conditions (pH = 0) to the pH of an aqueous ammonia solution (pH ![]() 11) therefore results in an increase of 0.650 volts in the half-cell potential for this reaction.
11) therefore results in an increase of 0.650 volts in the half-cell potential for this reaction.
The increase in the reducing strength of glucose when the reaction is run at pH 11 more than compensates for the decrease in oxidizing strength that results from the formation of the Ag(NH3)2 + complex ion. Thus, the overall cell potential for the reduction of silver ions to silver metal is actually more favorable in aqueous ammonia than under standard-state conditions.
![]()
Using the Nernst Equation to Measure Equilibrium Constants
The Nernst equation can be used to measure the equilibrium constant for a reaction. To understand how this is done, we have to recognize what happens to the cell potential as an oxidation-reduction reaction comes to equilibrium. As the reaction approaches equilibrium, the driving force behind the reaction decreases, and the cell potential approaches zero.
What implications does this have for the Nernst equation?
![]()
At equilibrium, the reaction quotient is equal to the equilibrium constant (Qc = Kc ) and the overall cell potential for the reaction is zero (E = 0).
| At equilibrium: | |
Rearranging this equation gives the following result.
| At equilibrium: | |
According to this equation, we can calculate the equilibrium constant for any oxidation-reduction reaction from its standard-state cell potential:
![]()
or, in a more useful arrangement:
![]()
This technique used in Practice Problem 10 can even be used to calculate equilibrium constants for reactions that don't seem to involve oxidation-reduction.
![]()
Harris Find Cu2 in a Solution Saturated
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch20/electro.php